Semaglutide and walking capacity in symptomatic PAD and type 2 diabetes patients: the STRIDE trial
Selected in The Lancet by Xavier Devoisin
This phase 3b, double-blind, randomised, placebo-controlled trial assessed the effect of semaglutide on walking capacity in patients with symptomatic peripheral artery disease (PAD) and type 2 diabetes.
After 52 weeks of treatment, semaglutide significantly improved maximal walking distance, symptoms, and quality of life compared to placebo. These results also suggest a potential anti-inflammatory effect of semaglutide, supporting its use in this high-risk population.
References:
Authors: Marc P Bonaca, Andrei-Mircea Catarig, Kim Houlind, Bernhard Ludvik, Joakim Nordanstig, Chethana Kalmady Ramesh, Neda Rasouli, Harald Sourij, Alex Videmark, Subodh Verma, STRIDE Trial Investigators
Reference: Lancet. 2025 Mar 28:S0140-6736(25)00509-4
DOI: 10.1016/S0140-6736(25)00509-4
Read the abstractObjective:
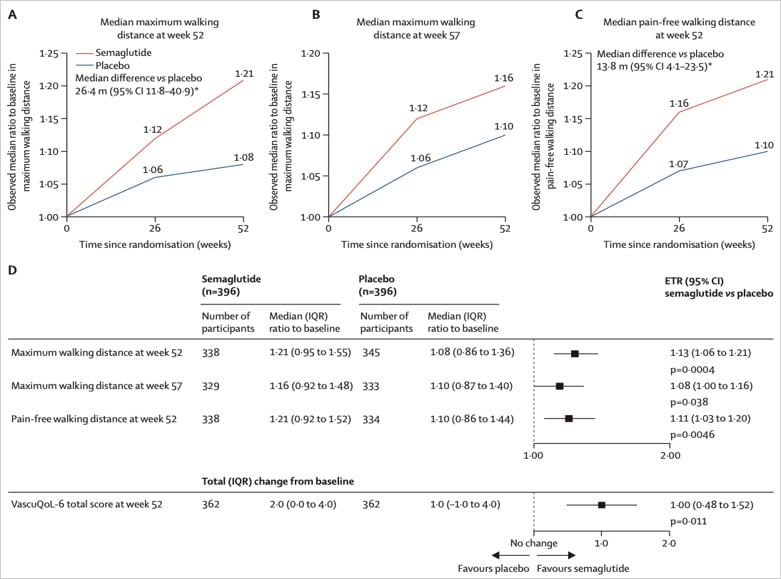
The objective was the comparison of maximum walking distance from baseline to week 52 — semaglutide vs placebo.
Study:
Phase 3b, double-blind, randomised, placebo-controlled clinical trial.
Population:
112 outpatient clinical trial centers, 20 countries in North America, Asia, and Europe
Inclusion: Type 2 Diabetes (T2D) + Stage IIA PAD (> 200m on flat treadmill and < 600m on inclined treadmill)
Outcomes and conclusion:
Significant improvement in function, symptoms, and quality of life, with a potential anti-inflammatory effect in patients with peripheral artery disease and type 2 diabetes.
Get the latest clinical cases and breaking news delivered straight to your inbox!


Comments:
Subcutaneous semaglutide 1 mg / week:
Key benefits:
Limitations:
Other considerations:
Weight loss and gastrointestinal effects may have revealed randomization, but unlikely to affect objective measures (walking distance or ABI)
Other available medications:
Both increase walking distance without impact on cardiovascular health