SWEDEPAD2: Paclitaxel-coated versus uncoated devices for infrainguinal endovascular revascularisation in patient with intermittent claudication
Selected in The Lancet by Basile Jaulin
SWEDEPAD 2 enrolled over 1,100 patients across 22 Swedish centres to evaluate the safety and effectiveness of paclitaxel-coated versus uncoated devices.
After one year, both groups demonstrated similar improvements in disease-specific quality of life, with no significant difference in major clinical outcomes.
However, a signal for increased late mortality beyond five years was observed in the paclitaxel group, echoing earlier concerns.
These results question the clinical benefit of paclitaxel-coated devices in claudicants and underscore the need for further investigation into their long-term safety.
References:
Authors: Prof Joakim Nordanstig, Prof Stefan James, Manne Andersson, Mattias Andersson, Martin Delle, Jan Engström, Torbjörn Fransson, Peter Gillgren, Anna Hilbertson, Tal M Hörer, Eva Jacobsson, Björn Kragsterman, Johan Lindbäck, Hans Lindgren, Karin Ludwigs, Stefan Mellander, Olle Nelzén, Robert Olin, Birgitta Sigvant, Per Skoog, Joachim Starck, Gustaf Tegler, Knut Thorbjørnsen, Maria Truedson, Prof Carl-Magnus Wahlgren, Jonas Wallinder, Andreas Öjersjö, Prof Mårten Falkenberg, on behalf of the SWEDEPAD trial investigators
Reference: Published August 30, 2025
DOI: DOI: 10.1016/S0140-6736(25)01584-3
Read the abstractBackground:
Peripheral artery disease (PAD) affects more than 230 million people worldwide and is responsible for intermittent claudication, tissue loss, and non-healing ulcers, with a major impact on quality of life, an aspect often underexplored in previous studies.
Endovascular revascularisation is the treatment of choice for symptomatic PAD after optimal medical therapy. Drug-coated balloons and stents using paclitaxel have demonstrated improved outcomes in reducing vessel recoil, restenosis, and ensuring long-term patency in vascular surgery.
However, their direct clinical benefit for patients remains insufficiently assessed, and most studies have included mixed populations of patients with intermittent claudication and critical limb ischaemia, complicating the interpretation of results.
Scientific background:
A 2018 meta-analysis by Katsanos et al. suggested an increased long-term mortality risk associated with paclitaxel-coated devices, sparking widespread concern and debate about their clinical safety and overall benefit.
Subsequent systematic reviews of the literature from 2000 to 2014 further questioned the true clinical advantage of drug-coated over uncoated devices. To address these uncertainties, two large randomised controlled trials—SWEDEPAD 1 and SWEDEPAD 2 (Swedish Drug-Elution Trial in Peripheral Arterial Disease)—were initiated to provide more robust evidence regarding the safety and efficacy of paclitaxel-based endovascular treatments.
Objective:
The SWEDEPAD 2 trial aimed to evaluate both the efficacy and safety of paclitaxel-coated devices compared with uncoated devices in infrainguinal endovascular revascularisation.
Beyond technical outcomes, the study focused on patient-centered endpoints, assessing symptom relief and improvement in quality of life, while specifically addressing concerns about potential long-term mortality associated with paclitaxel use.
Methods:
SWEDEPAD 2 was a large, multicentre, registry-based randomised controlled trial conducted across 22 of 28 Swedish centres using the national Swedvasc registry. Patients and statisticians were blinded, while operators were not. Randomisation (1:1) was computer-generated and stratified by centre after lesion crossing and before vessel preparation, with analysis performed on an intention-to-treat basis.
Eligible patients were ≥ 18 years old with intermittent claudication (Rutherford 1–3) due to ≥ 50 % infrainguinal stenosis or occlusion, suitable for endovascular treatment. Exclusion criteria included acute limb ischaemia, infrainguinal aneurysmal disease, previous participation in related trials, or an invalid Swedish personal ID number.
Between May 2014 and September 2023, 1,155 patients were randomised: 577 to paclitaxel-coated devices and 578 to uncoated devices. Median age was 73 years; 60 % had Rutherford class 3 disease, and 90 % were treated in the femoropopliteal segment.
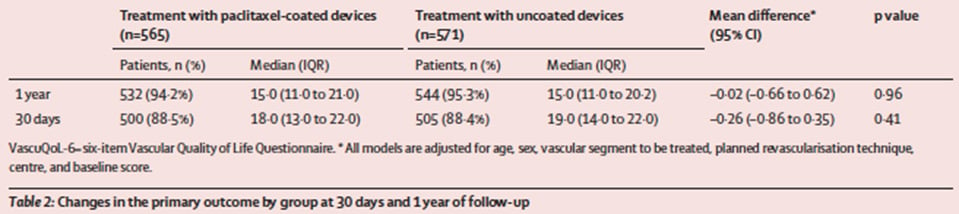
The primary endpoint was quality of life at one year, assessed using the validated VascuQoL-6 questionnaire (score range 6–24). Secondary endpoints included all-cause mortality, amputation-free survival, vessel patency, target lesion and limb reintervention rates, and ABI at one and five years.
Outcomes:
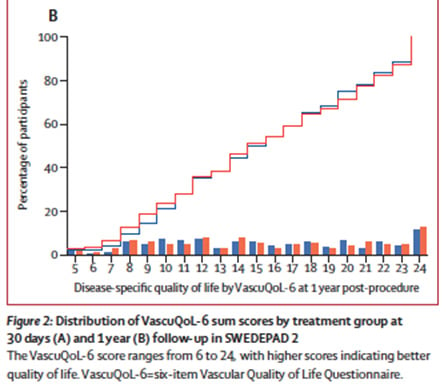
At one year, the VascuQoL-6 score improved significantly in both groups — from 10 to 15 — with no difference between paclitaxel-coated and uncoated devices (median score 15 in each group; p = n.s.). The evolution curves of quality-of-life scores were superimposable, and per-protocol results were consistent with the intention-to-treat analysis.
No significant differences were observed in ankle–brachial index or vessel patency at one year, as assessed by Doppler ultrasound.
Get the latest clinical cases and breaking news delivered straight to your inbox!


Discussion:
The SWEDEPAD 2 trial found no difference in quality-of-life improvement between paclitaxel-coated and uncoated devices, challenging the clinical benefit of paclitaxel in infrainguinal revascularisation. These results align with prior systematic reviews questioning its added value.
However, a late mortality signal at five years was observed in the paclitaxel group, echoing concerns raised in the 2018 meta-analysis, though not confirmed by SWEDEPAD 1.
Strengths of the study include its large, multicentre randomised design, strong external validity, blinded patients, and use of comprehensive national registries.
Limitations include operator unblinding, a relatively low randomisation rate, long inclusion period with evolving practices, reliance on subjective endpoints, and the absence of objective measures such as treadmill or six-minute walk tests.
Conclusion:
In patients with intermittent claudication, paclitaxel-coated balloons and stents showed no clinical benefit over uncoated devices in terms of disease-specific quality of life at one year, and were associated with a potential increase in late mortality beyond five years.
Reintervention rates were similar across both groups.
While SWEDEPAD 2 stands out for its robust, registry-based randomised design and long-term follow-up, operator unblinding and the use of subjective endpoints limit interpretation.
Further studies are warranted to clarify the long-term safety of paclitaxel devices and to identify any subgroup-specific effects.